03.13.19
Update: 3/10/2020: FDA Says 9 Out of 52 Cosmetic Products Tested Positive for Asbestos
The FDA issued a statement on March 4th, 2019 announcing new steps that it will take to better ensure the safety of cosmetic products.
The statement, issued by FDA Commissioner Scott Gottlieb, M.D. and Susan Mayne, Ph.D., director of the Center for Food Safety and Applied Nutrition, notes that the FDA's authority on overseeing cosmetics is limited -- and that the FD&C Act, which is the law governing the FDA’s oversight of cosmetic products, has not been updated since it was first enacted in 1938.
On March 12th, the House Subcommittee on Economic and Consumer Policy held a hearing, Economic and Consumer Policy: Examining the Public Health Risks of Carcinogens in Consumer Products.
At the hearing, experts discussed products containing talc. More about the hearing is below.
New Steps Outlined in the FDA's Statement
The FDA's statement urges cosmetic firms to “take responsible steps to voluntarily register their products and list ingredients, including talc, used in their products via the FDA’s Voluntary Cosmetic Registration Program.
The program provides a way for cosmetic manufacturers, distributors and packers to voluntarily file information on their products that are currently marketed to U.S. consumers. It also asks that companies register their manufacturing and/or packaging facility locations.
The FDA will also form an interagency working group to propose draft standards to improve analytical consistency for talc testing. This will be the subject of an upcoming public forum. The FDA also urges manufacturers to report adverse events involving cosmetic products through the FDA's MedWatch reporting system.
The statement says:
"We will be investigating how manufacturers source talc with appropriate traceability, and whether they test raw talc and/or their finished products. We also want to know how many cosmetics products contain talc and whether manufacturers have received adverse event reports associated with talc-containing products. We believe this information will help us better identify specific cosmetic products and raw ingredient suppliers that may be more likely to be contaminated and inform steps that the FDA may be able to take to better protect consumers."
Recalls & An Investigation Fuel the Talc Discussion
The new statement was drafted after the FDA's further review and assessment of talc-containing makeup products by Claire's and Justice stores that were found to contain asbestos in 2017.
After testing by a third-party lab in 2017, eight of Claire's makeup products and four Justice products were found to contain asbestos -- and both companies quickly removed the products from stores when they received a health advisory from the FDA.
Only Justice, however, issued a voluntary recall in 2017 -- prompting the FDA to conduct independent tests, which now confirm the presence of asbestos in one Justice product and three of Claire's products. The FDA issued this Safety Alert on March 5th, 2019, urging consumers to stop using those three Claire's products. The Alert was updated yesterday to note that Claire's announced a recall.
In a different case -- the Department of Justice and the Securities and Exchange Commission issued subpoenas in an investigation of asbestos in Johnson & Johnson's baby powder on February 21st, 2019.
FDA Says the Cosmetic Industry's Compliance is a Problem
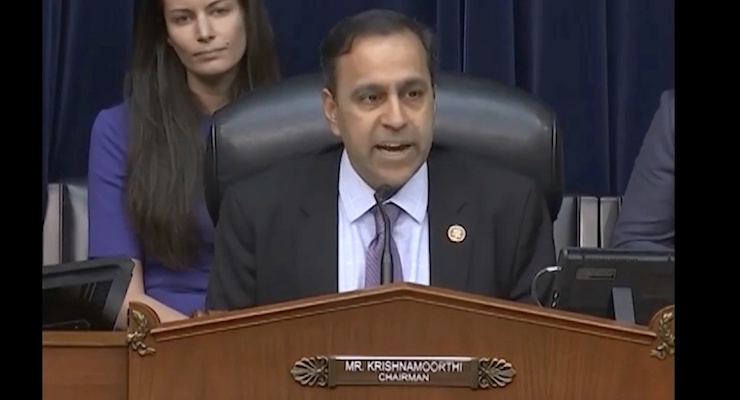
At yesterday's House Subcommittee hearing, "Economic and Consumer Policy: Examining the Public Health Risks of Carcinogens in Consumer Products, Chairman Krishnamoorthi delivered an opening statement that included the following remarks -- see the video below.
Krishnamoorthi said,
"...FDA authority generally remains weak...product recalls, mandatory risk labeling, and adverse event reports are just a few of the processes in which compliance with FDA guidelines is entirely voluntary for the cosmetics industry."
He continued, "This is a statutory problem that needs reform...But juries across America are not waiting for Congress to act."
Krishnamoorthi also noted that juries recently awarded verdicts to victims and survivors who suffered or died from ovarian cancer that was possibly caused by talcum baby powder -- emphasizing this point:
"Many of those juries have assessed punitive damages against manufacturers for failing to warn consumers of talcum powder's ovarian cancer risk."
He also stated that the average consumer in the U.S uses no less than 9 personal care products daily - and that consumers deserve to be protected from potentially carcinogenic products.
The hearing included statements from the Environmental Working Group, speaking about issues regarding the federal regulation of cosmetic products, and Johnson & Johnson, reporting on the safety of their products. Watch the opening statement below.
READ MORE
New Bill Introduced To Strengthen FDA's Oversight of Cosmetics